
Understanding Injection Molding in the Medical Field
Plastic injection molding is a manufacturing process where molten plastic material is injected into a precisely engineered mold cavity. Once cooled and solidified, the part is ejected, resulting in a finished component that requires minimal post-processing. In the medical context, this process occurs in controlled environments with specialized equipment to meet stringent industry requirements.
The medical industry demands manufacturing processes that deliver consistent quality, biocompatibility, and regulatory compliance. Injection molding has become the preferred method for producing everything from simple disposable components to complex surgical instruments and implantable devices.
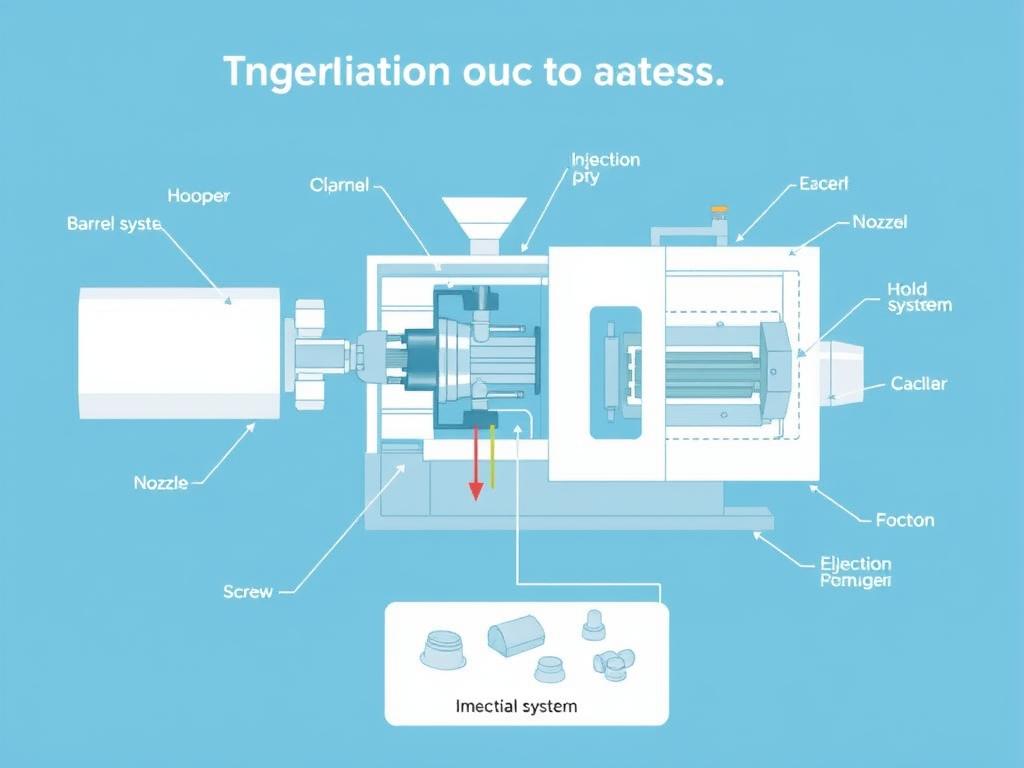
Key Advantages of Injection Molding for Medical Applications
Unmatched Precision and Consistency
Medical devices often require components with tolerances measured in micrometers. Injection molding delivers this precision consistently across production runs of thousands or millions of identical parts. This reliability is crucial for devices where even minor variations could impact functionality or patient safety.

Cost-Efficiency at Scale
While initial tooling costs can be significant, injection molding becomes increasingly cost-effective as production volumes rise. The automated nature of the process minimizes labor costs, while the speed of production and minimal material waste contribute to overall efficiency. For medical device manufacturers, this translates to more affordable healthcare solutions.

Material Versatility
Injection molding accommodates a wide range of medical-grade polymers, each offering specific properties suited to different applications. From rigid polycarbonates for durable equipment housings to flexible thermoplastic elastomers for seals and gaskets, this versatility enables customized solutions for diverse medical needs.

Design Flexibility
Complex geometries, integrated features, and variable wall thicknesses are all achievable through injection molding. This design freedom allows medical device engineers to create innovative solutions that would be impossible or prohibitively expensive with other manufacturing methods.

Common Applications of Injection Molding in Medical Field
The versatility of injection molding has made it indispensable across virtually every area of medical device manufacturing. From diagnostic tools to surgical instruments, this process delivers the precision and reliability healthcare demands.
Diagnostic Equipment
- Test cartridges and sample holders
- Analyzer housings and interfaces
- Microfluidic components
- Point-of-care testing devices
Surgical Instruments
- Handles and grips for precision tools
- Disposable surgical implements
- Endoscopic and laparoscopic components
- Sterilization trays and containers
Drug Delivery Systems
- Syringe bodies and components
- Inhaler housings and mechanisms
- Insulin pen components
- Implantable drug delivery devices
Implantable Devices
- Orthopedic spacers and components
- Cardiovascular device housings
- Neurological implant components
- Dental implant parts and accessories
Laboratory Equipment
- Pipette tips and dispensers
- Sample preparation devices
- Centrifuge components
- Storage and organization systems
Patient Care Products
- Monitoring device housings
- Fluid management components
- Respiratory care devices
- Rehabilitation equipment

Material Considerations for Medical Injection Molding
The selection of appropriate materials is critical in medical device manufacturing. FDA-approved plastics must meet stringent requirements for biocompatibility, sterilization compatibility, and mechanical properties.
| Material | Key Properties | Common Applications | Sterilization Methods |
| Polypropylene (PP) | Chemical resistance, fatigue resistance, translucent | Syringes, vials, laboratory equipment | Ethylene oxide, gamma radiation |
| Polycarbonate (PC) | Impact resistance, transparency, heat resistance | Device housings, surgical instruments, IV components | Autoclave, ethylene oxide, gamma radiation |
| Polyetheretherketone (PEEK) | High temperature resistance, chemical resistance, biocompatibility | Implantable devices, surgical instruments | Autoclave, ethylene oxide, gamma radiation |
| Acrylonitrile Butadiene Styrene (ABS) | Rigidity, impact resistance, surface finish | Equipment housings, device components | Ethylene oxide, gamma radiation |
| Medical-grade Silicone | Flexibility, biocompatibility, temperature resistance | Seals, gaskets, fluid delivery | Autoclave, ethylene oxide |
Sterilization Compatibility
Medical devices must withstand sterilization processes without degradation. Different materials respond differently to various sterilization methods:
- Autoclave (Steam): Requires materials with high heat resistance like PC, PEEK, and certain grades of PP
- Ethylene Oxide (EtO): Compatible with most medical-grade polymers
- Gamma Radiation: May cause discoloration or property changes in some materials
- E-beam Sterilization: Offers good material compatibility with controlled penetration depth
Material selection is not just about meeting physical requirements—it’s about ensuring patient safety through biocompatibility, chemical resistance, and sterilization compatibility across the device lifecycle.
— FDA Medical Device Guidelines
Challenges & Solutions in Medical Injection Molding
Regulatory Compliance
Medical device manufacturing is heavily regulated, with requirements varying by region and device classification. Injection molding for medical applications must adhere to standards like ISO 13485, FDA regulations, and EU MDR.
Successful manufacturers implement robust quality management systems that document every aspect of production, from material handling to final inspection. This comprehensive approach ensures traceability and compliance throughout the manufacturing process.
Contamination Control
Even microscopic contaminants can compromise device performance or patient safety. Medical injection molding typically occurs in cleanroom environments with controlled air quality, temperature, and humidity.
Advanced facilities implement automated handling systems, specialized equipment, and strict protocols to minimize human contact and potential contamination sources. Regular environmental monitoring ensures conditions remain within specified parameters.
Design Complexity
Medical devices often require complex geometries with tight tolerances. Advanced mold design, including sophisticated cooling systems and precision gating, helps achieve these demanding specifications.
Simulation software allows engineers to optimize designs before cutting steel, predicting potential issues like warpage, sink marks, or flow problems. This digital prototyping reduces development time and costs while improving first-article success rates.
Validation Requirements
Medical device manufacturing requires thorough validation of processes and equipment. The IQ/OQ/PQ (Installation Qualification, Operational Qualification, Process Qualification) framework ensures that production consistently meets specifications.
Implementing statistical process control (SPC) and automated inspection systems provides ongoing verification that parts remain within specification. This data-driven approach supports both quality assurance and continuous improvement efforts.

Future Trends in Medical Injection Molding
The intersection of medical needs and manufacturing technology continues to evolve, driving innovations in injection molding for healthcare applications.
Micro-Injection Molding
As medical devices become smaller and more precise, micro-injection molding enables the production of components with features measured in micrometers. This technology supports minimally invasive procedures, implantable sensors, and advanced drug delivery systems.
Biodegradable Materials
Environmentally friendly and bioabsorbable polymers are gaining traction for temporary implants and single-use devices. These materials perform their function and then safely degrade, eliminating the need for removal procedures and reducing medical waste.
Multi-Material Molding
Advanced techniques like overmolding and two-shot molding combine different materials in a single component. This enables complex functionality, such as rigid structures with soft-touch surfaces or integrated seals, reducing assembly steps and potential failure points.
Conclusion: The Future of Medical Manufacturing
Injection molding in medical field continues to evolve, enabling innovations that were unimaginable just decades ago. As healthcare demands more personalized, precise, and cost-effective solutions, this manufacturing process remains at the forefront of medical device development.
The combination of advanced materials, precision engineering, and stringent quality controls makes injection molding uniquely suited to address the complex challenges of modern healthcare. From life-saving implantable devices to diagnostic tools that detect disease earlier than ever before, injection molded components play a critical role in advancing medical care worldwide.
For medical device manufacturers, mastering the intricacies of this process—from material selection to regulatory compliance—is essential for bringing innovative healthcare solutions to market efficiently and effectively.
Ready to Transform Your Medical Device Manufacturing?
Our team of specialists combines decades of experience in medical injection molding with cutting-edge technology to deliver solutions that meet the highest standards of quality and compliance. Contact us now!

